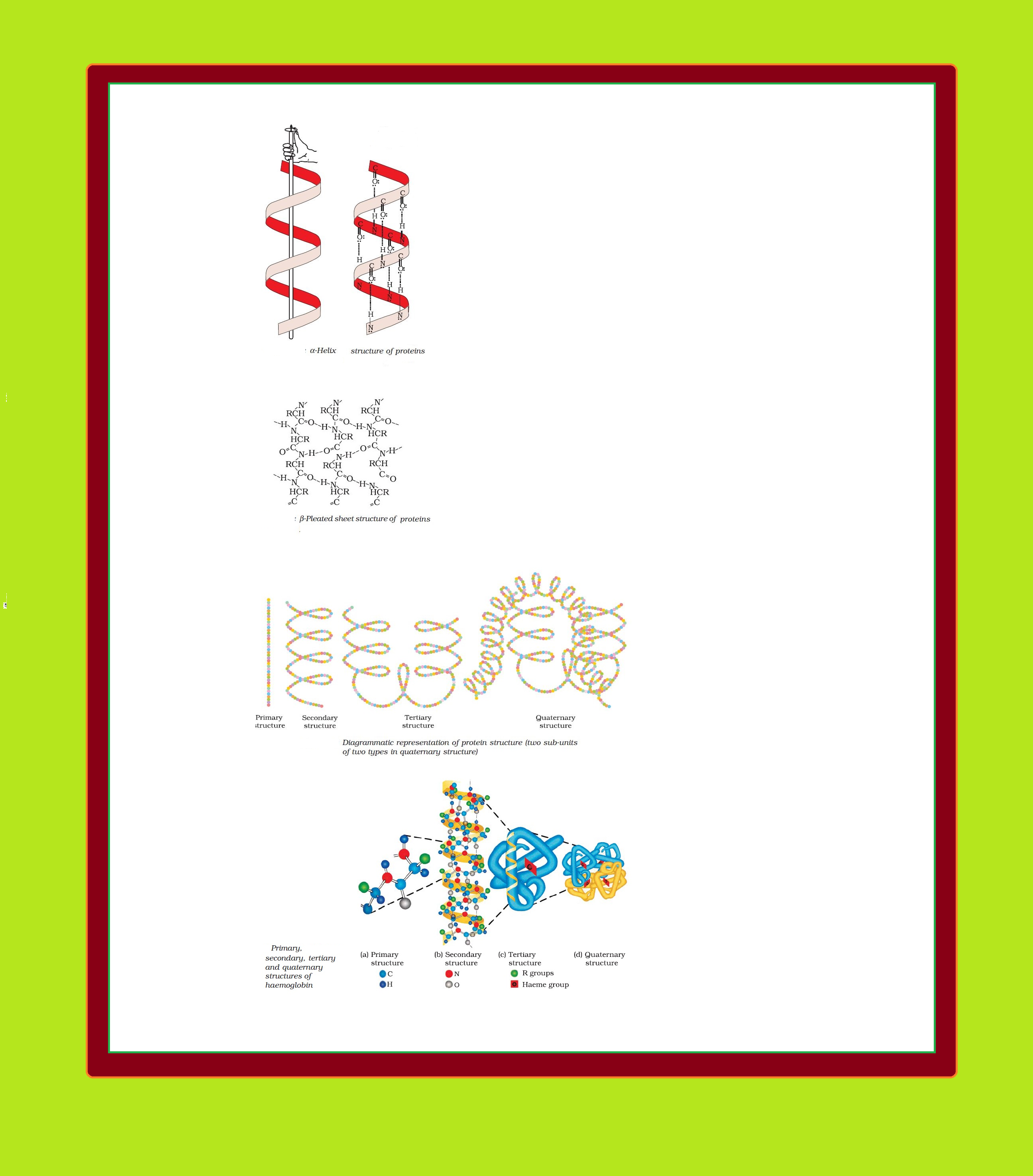
Structure of Proteins :
`=>` Proteins are the polymers of `color{red}(α)`-amino acids and they are connected to each other by peptide bond or peptide linkage.
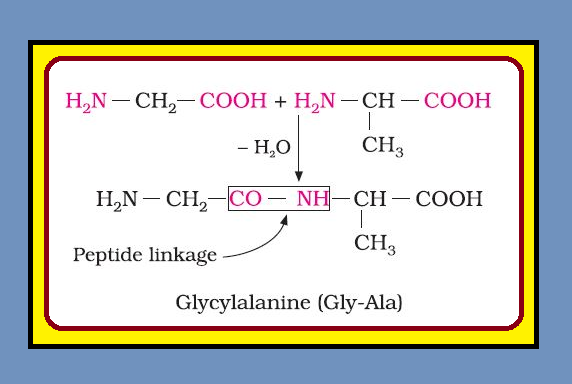
`=>` Chemically, peptide linkage is an amide formed between `color{red}(–COOH)` group and `color{red}(–NH_2)` group.
`=>` The reaction between two molecules of similar or different amino acids, proceeds through the combination of the amino group of one molecule with the carboxyl group of the other.
`=>` This results in the elimination of a water molecule and formation of a peptide bond `color{red}(–CO–NH–)`. The product of the reaction is called a dipeptide because it is made up of two amino acids. For example, when carboxyl group of glycine combines with the amino group of alanine we get a `color{green}("dipeptide")`, glycylalanine.
● If a third amino acid combines to a dipeptide, the product is called a `color{green}("tripeptide")`.
● A tripeptide contains three amino acids linked by two peptide linkages.
● Similarly when four, five or six amino acids are linked, the respective products are known as `color{green}("tetrapeptide")`, `color{green}("pentapeptide")` or `color{green}("hexapeptide")`, respectively.
● When the number of such amino acids is more than ten, then the products are called `color{green}("polypeptides")`.
● A polypeptide with more than hundred amino acid residues, having molecular mass higher than `10,000u` is called a `color{green}("protein")`.
● The distinction between a polypeptide and a protein is not very sharp.
● Polypeptides with fewer amino acids are likely to be called proteins if they ordinarily have a well defined conformation of a protein such as insulin which contains `51` amino acids.
`=>` Proteins can be classified into two types on the basis of their molecular shape.
`=>` Chemically, peptide linkage is an amide formed between `color{red}(–COOH)` group and `color{red}(–NH_2)` group.
`=>` The reaction between two molecules of similar or different amino acids, proceeds through the combination of the amino group of one molecule with the carboxyl group of the other.
`=>` This results in the elimination of a water molecule and formation of a peptide bond `color{red}(–CO–NH–)`. The product of the reaction is called a dipeptide because it is made up of two amino acids. For example, when carboxyl group of glycine combines with the amino group of alanine we get a `color{green}("dipeptide")`, glycylalanine.
● If a third amino acid combines to a dipeptide, the product is called a `color{green}("tripeptide")`.
● A tripeptide contains three amino acids linked by two peptide linkages.
● Similarly when four, five or six amino acids are linked, the respective products are known as `color{green}("tetrapeptide")`, `color{green}("pentapeptide")` or `color{green}("hexapeptide")`, respectively.
● When the number of such amino acids is more than ten, then the products are called `color{green}("polypeptides")`.
● A polypeptide with more than hundred amino acid residues, having molecular mass higher than `10,000u` is called a `color{green}("protein")`.
● The distinction between a polypeptide and a protein is not very sharp.
● Polypeptides with fewer amino acids are likely to be called proteins if they ordinarily have a well defined conformation of a protein such as insulin which contains `51` amino acids.
`=>` Proteins can be classified into two types on the basis of their molecular shape.